
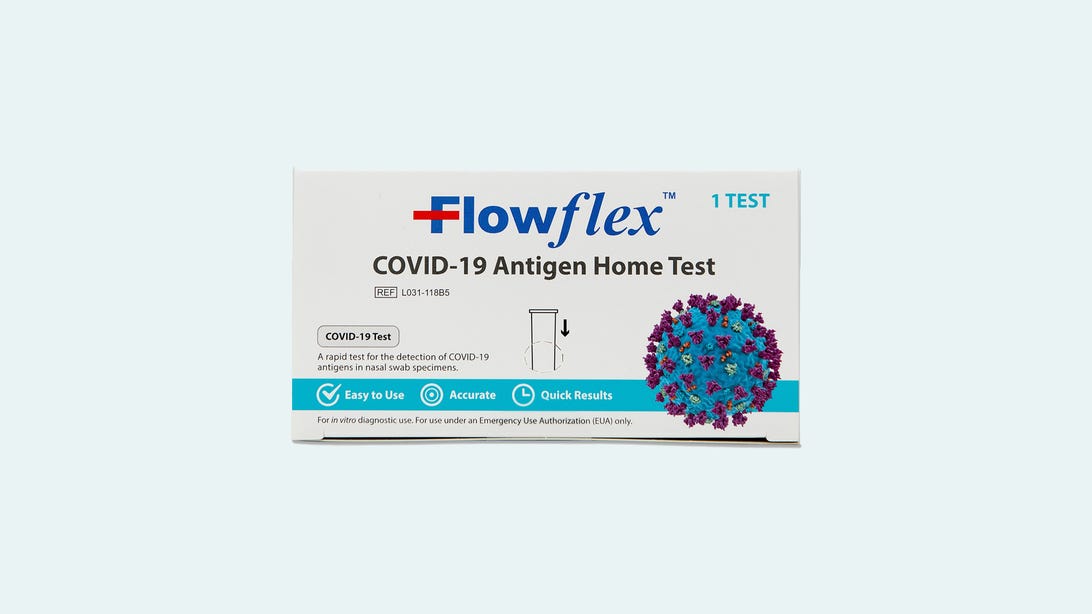
Flowflex tests that look like this are authorized and available in the US.
Flowflex
If you have an at-home COVID-19 Flowflex test that came in a dark blue box, don’t use it, the US Food and Drug Administration said this month. At least, if you got it in the US.
While the Flowflex test that comes in a white box with the full name “Flowflex COVID-19 Antigen Home Test” is safe to use (it has an emergency use authorization from the FDA), there’s a risk of counterfeit tests being sold in the US with a blue box and a slightly different name, “Flowflex SARS-CoV-2 Antigen Rapid Test (Self-Testing),” according to a company recall by the maker of the authorized Flowflex tests, ACON Laboratories.
The tests being recalled come in a blue box and are manufactured by ACON Biotech (Hangzhou) Co., Ltd, which can be seen on the back of the box.
Blue-boxed Flowflex COVID-19 tests are legally available in Europe under the same name because they’re regulated there. But the same test cannot be “cannot be legally imported, distributed, or used” in the US and should be reported, according to the announcement.
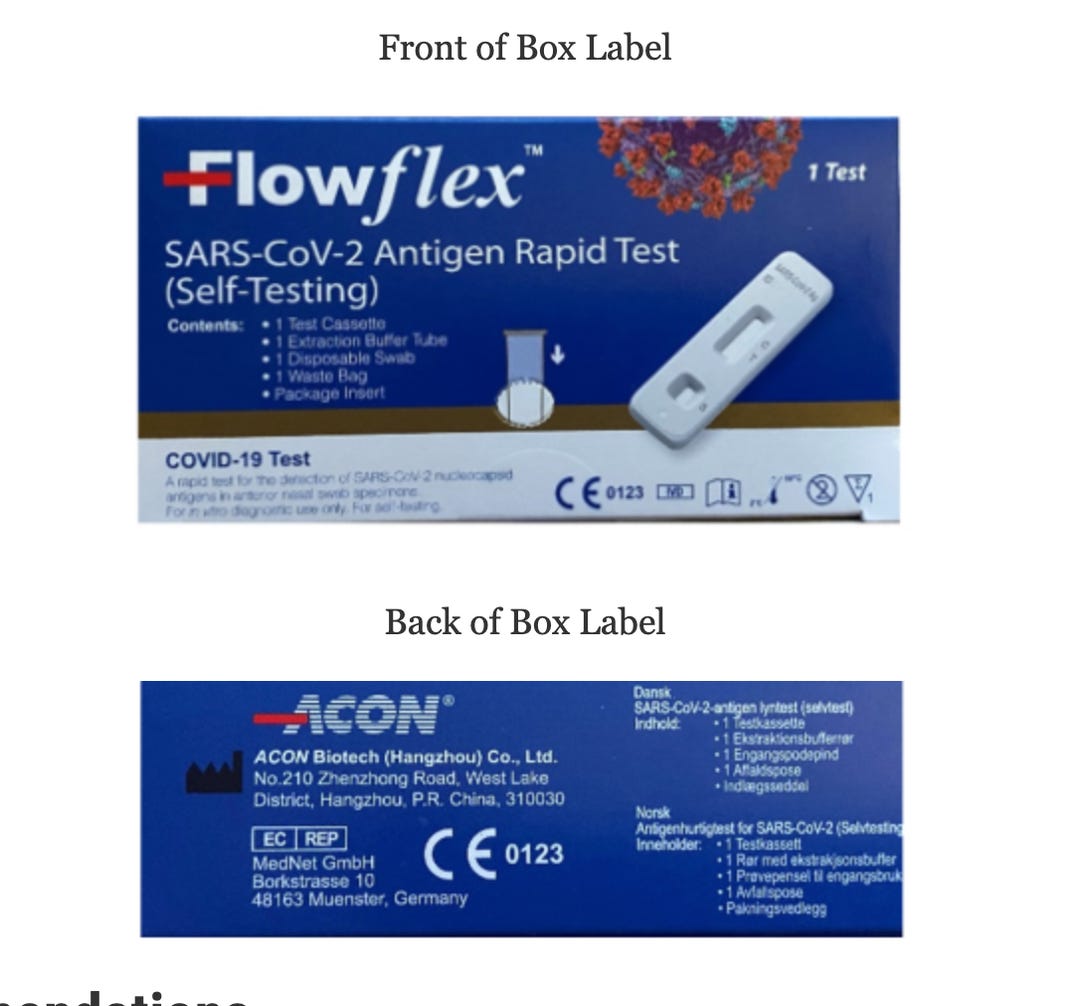
If your Flowflex test box looks like this, it’s being recalled.
FDA
The company made the announcement about the other Flowflex tests in January, and the FDA published it on Friday.
There’ve been no reports of adverse events, but because the duplicate tests haven’t been tested or authorized by the FDA, there’s a possibility of a false negative or false positive result. If you’ve had a COVID-19 test with one of the recalled antigen tests within two weeks, consider testing again, the FDA said.
If it’s been more than two weeks since you’ve used one of the tests and you don’t have any symptoms, it’s not necessary to test again, per the FDA.
All distribution of the recalled Flowflex tests in the US should be reported to ACON and the FDA, according to the announcement. Information on how to contact ACON and the FDA can be found at the bottom of the recall announcement.
Read more: How to Make Sure Your COVID Test Isn’t Fake
The information contained in this article is for educational and informational purposes only and is not intended as health or medical advice. Always consult a physician or other qualified health provider regarding any questions you may have about a medical condition or health objectives.