
Barbara Campbell was walking through a New York City subway station during rush hour when her world abruptly went dark. For four years, Campbell had been using a high-tech implant in her left eye that gave her a crude kind of bionic vision, partially compensating for the genetic disease that had rendered her completely blind in her 30s. “I remember exactly where I was: I was switching from the 6 train to the F train,” Campbell tells IEEE Spectrum. “I was about to go down the stairs, and all of a sudden I heard a little ‘beep, beep, beep’ sound.”
It wasn’t her phone battery running out. It was her Argus II retinal implant system powering down. The patches of light and dark that she’d been able to see with the implant’s help vanished.
Terry Byland is the only person to have received this kind of implant in both eyes. He got the first-generation Argus I implant, made by the company
Second Sight Medical Products, in his right eye in 2004 and the subsequent Argus II implant in his left 11 years later. He helped the company test the technology, spoke to the press movingly about his experiences, and even met Stevie Wonder at a conference. “[I] went from being just a person that was doing the testing to being a spokesman,” he remembers.
Yet in 2020, Byland had to find out secondhand that the company had abandoned the technology and was on the verge of going bankrupt. While his two-implant system is still working, he doesn’t know how long that will be the case. “As long as nothing goes wrong, I’m fine,” he says. “But if something does go wrong with it, well, I’m screwed. Because there’s no way of getting it fixed.”
Ross Doerr, another Second Sight patient, doesn’t mince words: “It is fantastic technology and a lousy company,” he says. He received an implant in one eye in 2019 and remembers seeing the shining lights of Christmas trees that holiday season. He was thrilled to learn in early 2020 that he was eligible for software upgrades that could further improve his vision. Yet in the early months of the COVID-19 pandemic, he heard troubling rumors about the company and called his Second Sight vision-rehab therapist. “She said, ‘Well, funny you should call. We all just got laid off,’ ” he remembers.
“She said, ‘By the way, you’re not getting your upgrades.’ ”
These three patients, and more than 350 other blind people around the world with Second Sight’s implants in their eyes, find themselves in a world in which the technology that transformed their lives is just another obsolete gadget. One technical hiccup, one broken wire, and they lose their artificial vision, possibly forever. To add injury to insult: A defunct Argus system in the eye could cause medical complications or interfere with procedures such as MRI scans, and it could be painful or expensive to remove.

Barbara Campbell’s retinal implant abruptly powered down during a subway transfer in Manhattan, and never worked again. Beatrice de Gea/The New York Times/Redux
Neural implants—devices that interact with the human nervous system, either on its periphery or in the brain—are part of a rapidly growing category of medicine that’s sometimes called electroceuticals. Some technologies are well established, like deep-brain stimulators that reduce tremors in people with Parkinson’s disease. But recent advances in neuroscience and digital technology have sparked a gold rush in brain tech, with the outsized investments epitomized by Elon Musk’s buzzy brain-implant company, Neuralink. Some companies talk of reversing depression, treating Alzheimer’s disease, restoring mobility, or even dangle the promise of superhuman cognition.
Not all these companies will succeed, and Los Angeles–based Second Sight provides a cautionary tale for bold entrepreneurs interested in brain tech. What happens when cutting-edge implants fail, or simply fade away like yesterday’s flip phones and
Betamax? Even worse, what if the companies behind them go bust?
After Second Sight discontinued its retinal implant in 2019 and nearly went out of business in 2020,
a public offering in June 2021 raised US $57.5 million at $5 per share. The company promised to focus on its ongoing clinical trial of a brain implant, called Orion, that also provides artificial vision. But its stock price plunged to around $1.50, and in February 2022, just before this article was published, the company announced a proposed merger with an early-stage biopharmaceutical company called Nano Precision Medical (NPM). None of Second Sight’s executives will be on the leadership team of the new company, which will focus on developing NPM’s novel implant for drug delivery.
The company’s current leadership declined to be interviewed for this article but did provide an emailed statement prior to the merger announcement. It said, in part: “We are a recognized global leader in neuromodulation devices for blindness and are committed to developing new technologies to treat the broadest population of sight-impaired individuals.”
Spectrum pieced together Second Sight’s story by interviewing half a dozen patients, a company cofounder, and eight doctors or researchers involved with the company. In their telling, the company took hundreds of patients on a roller-coaster ride of technological innovations, regulatory successes, medical and financial setbacks, and a near-total meltdown. Now, as the company fades away, the future of high-tech vision implants seems blurrier than ever.
Second Sight began with a flash of light. In 1991, Robert Greenberg, an electrical engineer turned medical student, stood in an operating room watching as a retinal surgeon inserted a tiny wire into the eye of a blind patient, who was awake and under local anesthesia. When the wire touched the patient’s retina and delivered a minuscule jolt of electric current, the man reported a spot of light in his pitch-black field of vision. The surgeon inserted a second wire, and the man saw two spots of light. “If you can create two spots, it seemed obvious to me that you could do a lot of spots and create images,” Greenberg said in a 2011 interview for an Spectrum article. “We just needed to build a device.”

Robert Greenberg cofounded Second Sight and worked for years to bring the retinal implants to market. Ringo Chiu/ZUMA Press/Alamy
Of course, it wasn’t that easy. Greenberg spent many years developing the technology while working at the
Alfred Mann Foundation, a nonprofit organization that develops biomedical devices; he spun off the company Second Sight with three cofounders in 1998. The clinical trials of the first-generation Argus I (with a 16-electrode array) and the subsequent Argus II retinal implant (with a 60-pixel array) resulted in European regulatory approval in 2011 and U.S. approval in 2013.
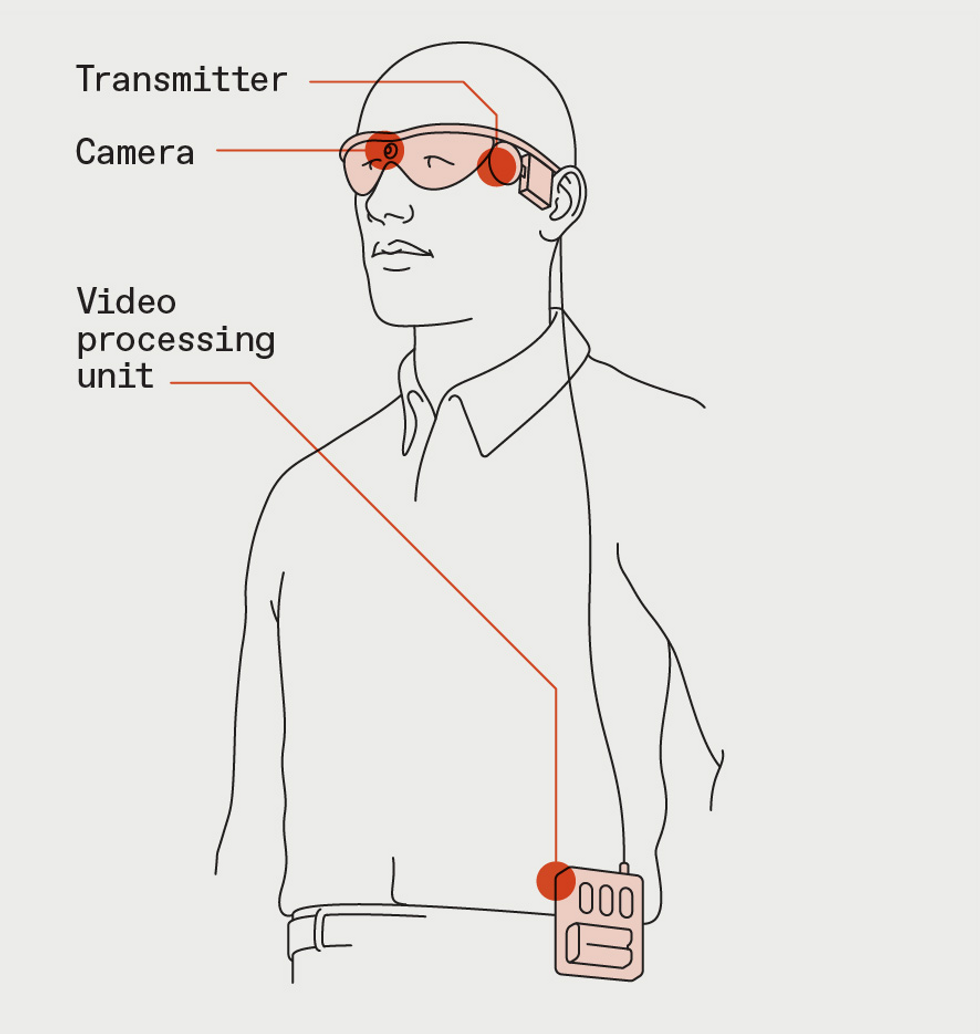
Second Sight’s two technologies for artificial vision both start with a camera that streams video to a video processing unit. The VPU converts the images to simple patterns of 60 pixels and sends that information to a transmitter on the user’s glasses.James Provost
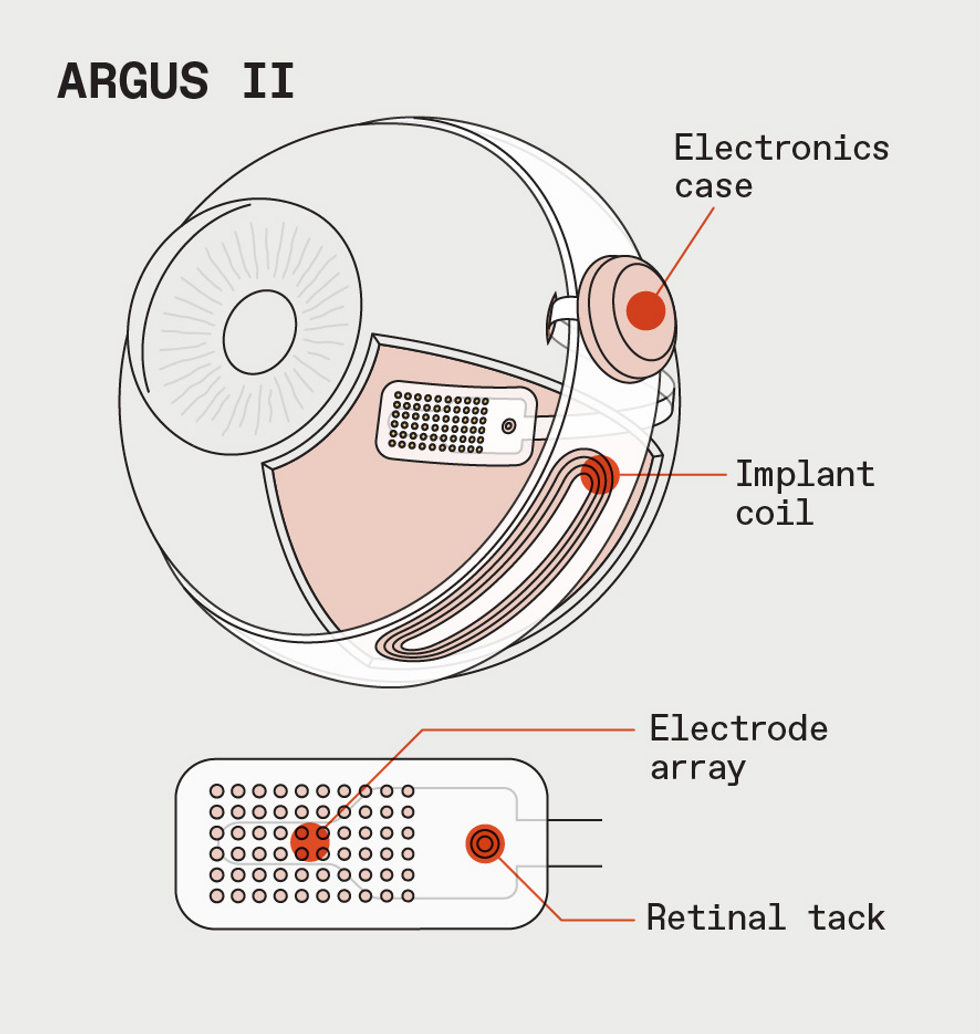
For Argus II, the stimulation patterns go to an implant in the retina.James Provost
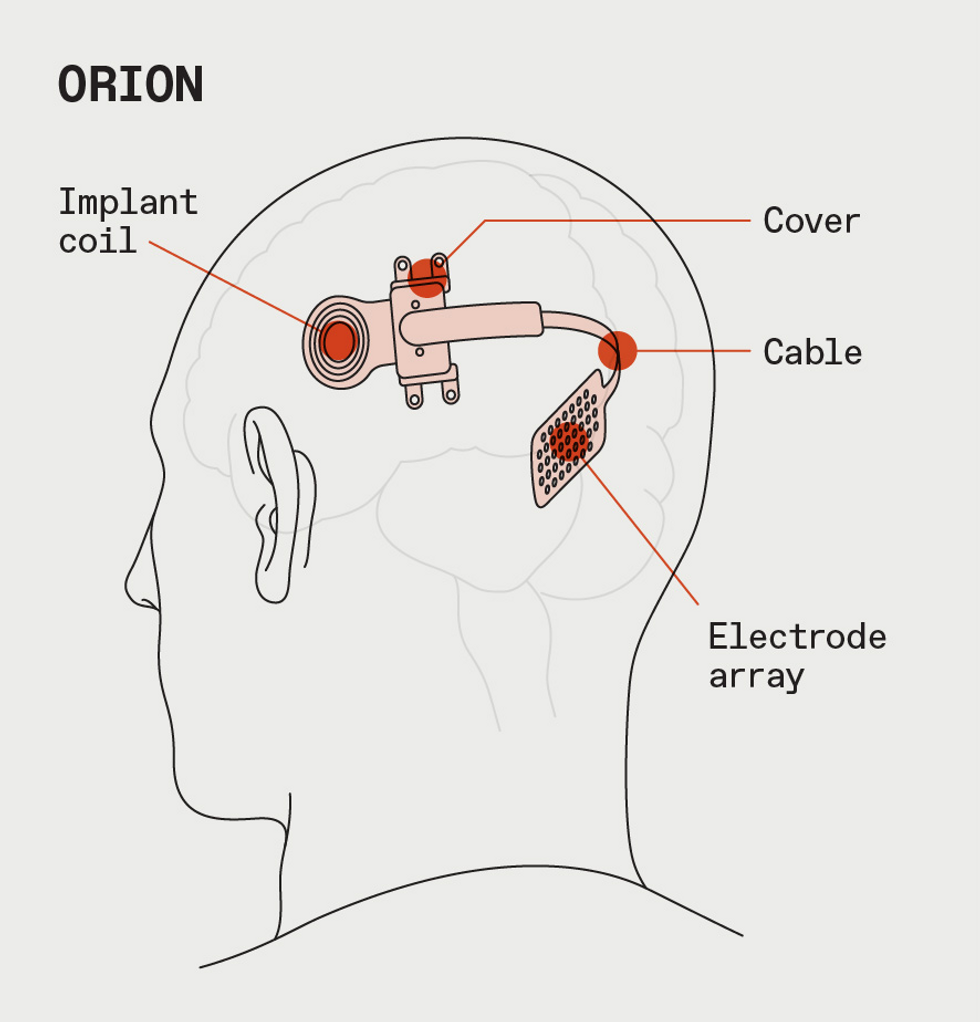
For Orion, which is now in clinical trials, the stimulation patterns go to an implant in the brain.James Provost
The Argus II system consists of more than just the implant, which is surgically implanted in a procedure that takes about 4 hours. The user also wears special glasses outfitted with a small camera that sends video down a wire to a video processing unit (VPU), typically attached to the user’s belt.
The VPU reduces the images to patterns of 60 black-and-white pixels and sends them back to a transponder in the glasses, which beams them wirelessly to an antenna on the outside of the eye. From there, the signal goes to the 60-electrode array attached to the patient’s retina. The electrodes stimulate the eye in different patterns multiple times per second, creating flashes of light that correspond to the low-resolution video feed. Essentially, the electrodes take the place of the photoreceptor cells in a healthy eye that respond to light and send information up the optic nerve to the brain.
Normal vision, this is not. Patients and doctors alike stress that the Argus II provides a kind of artificial vision, really a brand-new sense that people must learn how to use. Argus II users perceive shades of gray that appear and disappear as they move their heads. “This was the first of its kind, it was a fledgling technology,” Greenberg told
Spectrum in a recent interview. “We asked ourselves a lot: What’s good enough? There’s no doubt that it was very crude.” In its e-mailed statement, Second Sight says that most patients saw well enough to assist with basic locomotion.
But the company admits that the results varied. While some patients could make out the white stripes of a crosswalk against a dark road or the brightness of a face turned toward them, others struggled to see even basic patterns and shapes. Still, for many, it was worth it. Doerr remembers his attitude before the surgery: “Although it isn’t normal vision, it’s 100 percent better than what I have now.”
Jeroen Perk, who lives in the Netherlands, lost his sight almost completely by the age of 19. In 2013, at the age of 36, Perk became one of the youngest people to receive an Argus II. He was a success story: Within just a couple of years, Perk was shown in Second Sight videos
skiing and shooting arrows.
Jeroen Perk used his Argus II retinal implant to try archery and skiing.
Lucian Del Priore was one of the physicians involved in the clinical trials; he did the implantation for Barbara Campbell while at NewYork-Presbyterian, in New York City. He remembers the excitement when the U.S. Food and Drug Administration approved the Argus II technology for people with a genetic condition called retinitis pigmentosa, and notes that there were no other options for such patients.
“I’m a lucky bastard. I never have any regrets about doing this.” —Jeroen Perk
“These people were completely in the dark,” he says. “They couldn’t tell the difference between a bright day at the beach and being in a coal mine in Pittsburgh. The idea that they were getting some kind of vision, it was kind of electrifying—for the patients and the doctors.”
While the Argus II was technically impressive, it faced financial headwinds. Second Sight was selling the Argus II for around $150,000 in the United States—about five times as much as other neuromodulation devices, according to Greenberg. But even so, he says, the company was losing money: “With all the overhead of sales and regulatory people, it wasn’t profitable.”
Moreover, implanting the Argus II was just the start of a long, tough journey for patients. Second Sight employed its own vision-rehabilitation specialists to work one-on-one with implantees, often for months or years. One Argus II patient estimated that the total cost of the device, surgery, and rehabilitation was $497,000. Typically, at least 80 percent of the device fee and most of the other costs were covered by insurance.
A positive outcome was far from certain. Although Linda Kirk was the subject of an
optimistic news story on receiving her Argus II in New York in 2017, she found the implant more distracting than enabling. “I really wanted to be able to tell them, this is great; it’s a success. And I couldn’t do that,” she tells Spectrum. Kirk stopped using the device a couple of years later.
At the nonprofit
Lighthouse International, a senior fellow in vision science named Aries Arditi was a principal investigator for the Argus II clinical trial. Arditi says his experiences with patients slowly soured him on the technology. He tells Spectrum that in his decades of work with people who were born sighted and later lost their vision, he’s learned that “they often develop a desperate hope for something that will help and are willing to try anything.” Arditi feels that Second Sight promised more than it delivered. “I found it very disturbing that [Second Sight] sold so many of these devices to patients who were relying on hope rather than proven performance.”
Arditi also says that he did a research study including nearly all the U.S. participants in the Argus II clinical trial that showed “weakness” with the device’s vision quality. He says Second Sight wouldn’t let him publish or present the results; the company says it disagreed with his analysis and discouraged him from publishing.
Barbara Campbell, who received her implant during the clinical trial of the Argus II, did find the bionic vision system useful. As a New York City resident, she used it outside on the busy sidewalks and while taking a subway or bus. “The more I used it, the benefits increased,” she remembers. “I think I was retraining my brain to see stuff.” But in 2013, after four years of regular use, Campbell’s system shut down in the subway station, and despite some repair attempts by Second Sight, never worked again. While she talked with her doctors about having the implant removed, she ultimately decided that the risks of another surgery weren’t worth it. She still has the defunct technology in her left eye.
Simply not working isn’t the worst thing that could happen—there’s also the possibility of medical problems. Second Sight conducted an FDA-mandated postapproval study of the Argus II, following 30 patients from 2007 to 2019. Over that time, 36 serious and 152 nonserious “adverse events” were observed. The FDA did not make the study’s final report available to Spectrum, and a Freedom of Information Act request filed in May 2021 has yet to be fulfilled.
However, the FDA also maintains a public database called Manufacturer and User Facility Device Experience, or
MAUDE, where manufacturers are required (and health care professionals and patients are encouraged) to submit reports of serious adverse events.
Spectrum analyzed all 90 MAUDE reports for the Argus II, submitted from 2014 to 2020. These reports are unverified and could be duplicate, biased, inaccurate, or incomplete, warns the FDA. While some described inflammation, infection, or pain that could be managed with medication, nearly 80 percent reported a surgical intervention. The reasons for surgery included hemorrhages, low eye pressure and, in about 15 percent of cases, detached retinas.
To be sure, such outcomes were not very common. Data from 2017 (
published in 2020) showed that 83 percent of 244 postapproval patients had experienced no serious events after two years.
“I felt like we were on the verge of really making a big breakthrough.” —Terry Byland
Terry Byland did not encounter any problems with his 2004 Argus I implant. In fact, when Second Sight offered him the Argus II, he was eager to try it. “Once you get a taste of being able to see certain things again, you want to continue on and make it better,” he tells Spectrum. By June 2015, Byland was the only person on the planet with two bionic eyes.
The jump from the Argus I’s 16 electrodes to 60 in the newer technology improved Byland’s vision, and it seemed like more advances were just around the corner. During a series of tests at the University of Southern California and Second Sight in 2016 and 2017, Byland was told about “virtual electrodes,” that is, software upgrades that would boost his system fourfold to around 250 pixels, as well as a new video processing unit. “I was sold,” he says. “I felt like we were on the verge of really making a big breakthrough.”
Other Argus II patients
Spectrum spoke with were also told they would be getting upgrades, such as a digital camera, thermal imaging, and even facial recognition software. In 2016, a USC professor even raised the possibility of color vision.
By 2018, Byland’s impressions had shifted. Second Sight continued to ask him to do promotional visits, but testing had slacked off—and there was no sign of any new technology. “It just felt like maybe somebody there wasn’t being completely honest with me,” he says.
Company cofounder Greenberg says Second Sight’s long-term plan was always to pivot to a brain implant that would bypass the eye altogether and directly stimulate the visual cortex. A neural device could help more people with vision problems, even those who weren’t eligible for an Argus II implant because of severe damage to the retina or optic nerve. But Greenberg wasn’t able to steer the company through that transition.
Greenberg’s relationship with Second Sight’s investors had been worsening over the years; he stepped down as CEO in 2015 and then
left the board of directors in 2018, a move that he has characterized as a forced departure but that he declined to discuss with Spectrum because of a non disclosure agreement (NDA).
On 18 July 2019, Second Sight sent Argus patients a letter saying it would be phasing out the retinal implant technology to clear the way for the development of its next-generation brain implant for blindness, Orion, which had begun a clinical trial with six patients the previous year. The U.S. National Institutes of Health is funding that trial as a
$6.4 million project over five years.
“The leadership at the time didn’t believe they could make [the Argus] part of the business profitable,” Greenberg says. “I understood the decision, because I think the size of the market turned out to be smaller than we had thought.”
Second Sight was quick to assure Argus patients that its support of their retinal devices would not change. “Second Sight will be maintaining a full team of Customer Care and Vision Rehab Specialists accessible to you as we have in the past,” read the letter. “In addition, we have taken all measures to ensure the ongoing supply needs for your device.”
However, the letter’s promises were already looking shaky, according to one ex-engineer at the company, who asked not to be named because they had also signed an NDA. “We didn’t really support the basic Argus after that,” the engineer tells Spectrum. “We didn’t sell any more, we didn’t make any more, we didn’t have anything to do with it anymore.”
And worse was yet to come, for Argus and Orion patients alike.
In February 2020, the senior director of implant R&D left the company, swiftly followed by its CEO. On 30 March, Second Sight laid off the majority of its remaining employees and announced its “
intention to wind down operations,” citing the impact of the COVID-19 pandemic on its ability to secure financing. Within weeks, most of its physical assets—including manufacturing equipment, scientific instruments, laptops, and shelving—went up for sale at auction.

Ross Doerr couldn’t get an MRI to check for a brain tumor because his doctors couldn’t get information about his implant from Second Sight. Bob O’Connor
Second Sight didn’t inform any of its patients of the company’s collapse. “No letter, email, or telephone call,” Ross Doerr wrote on Facebook after weeks of fruitlessly trying to contact the company. “Those of us with this implant are figuratively and literally in the dark.”
The implications of Second Sight’s implosion would soon strike home for Doerr. While using the Argus II for more than a few hours had always caused him to feel a little dizzy—a well-known side effect—early in 2020 he started to experience severe vertigo.
Doerr’s doctor scheduled an MRI scan to rule out a brain-stem tumor. But because an MRI’s intense magnetic fields can interact with the Argus II, MRI providers are instructed to contact Second Sight before performing any scans—and Second Sight wasn’t picking up the phone. Doerr eventually got a CT scan instead, which found nothing. “I still don’t know if I have a brain-stem tumor or not,” he tells
Spectrum.
Jeroen Perk also suffered from the transition. A regular user of the Argus II for up to 9 hours a day, Perk was using the system in November 2020 when the video processing unit (VPU) fell from his belt to the ground and shattered. “I had no vision, no Argus, and no support from Second Sight,” he remembers.
For a week Perk considered his options, including having the device removed from his retina. “My conclusion was, I must have [my vision] back,” he says. Perk shared his situation with the Argus II community in Europe, asking if anyone had spare parts. He quickly heard back from a patient who was no longer using the device and from a doctor with a spare VPU. By February 2021, he had a refurbished system, and Perk is now happily using it.
Jeroen Perk’s Argus II system stopped working when he dropped his video processing unit. He had to crowdsource spare parts to get the system working again. Jeroen Perk
“It is a pity that the Argus is not going any further,” he says. “But I’m a lucky bastard. I never have any regrets about doing this.”
In its statement to
Spectrum, Second Sight says that during its financial difficulties, its reduced workforce “was unable to continue the previous level of support and communication for Argus II centers and users.” After Spectrum contacted Second Sight, the company sent letters to Argus clinicians and users stating, “we will do our best to provide virtual support” to physicians and that it has a limited supply of VPUs and glasses for replacements. No repairs or replacements are possible, however, for the implants themselves.
It’s unclear what Second Sight’s proposed merger means for Argus patients. The day after the merger was announced, Adam Mendelsohn, CEO of Nano Precision Medical, told
Spectrum that he doesn’t yet know what contractual obligations the combined company will have to Argus and Orion patients. But, he says, NPM will try to do what’s “right from an ethical perspective.” The past, he added in an email, is “simply not relevant to the new future.”
Even clinicians were taken by surprise by Second Sight’s collapse in 2020. “It’s not something that we talked to any of the patients about, because I don’t think it crossed any of our minds,” says Andre Witkin, director of clinical research at Tufts Medical Center.
Neurosurgeon
Nader Pouratian, now at UT Southwestern Medical Center, is one of the researchers involved in the clinical trial of Second Sight’s Orion brain implant. “Up until days before Second Sight announced that it was reorganizing, we were actively planning the next stages of the research,” he says. Pouratian says the Orion patients were worried about having unsupported devices left in their brains: “There was a lot of anxiety among some patients that they were going to be left high and dry.”
Benjamin Spencer is one of the six Orion patients. He had been blind for 26 years when he received his neural implant in 2018, and he was initially delighted with it. When he first used it at home with his family, “it was amazing,” he says. “Here’s my wife—I’ve never seen [her] other than in my dreams.” He tells
Spectrum that the Orion enabled him to walk through a grocery store and do his shopping without using a cane.
Spencer’s feelings about the device shifted when he heard that Second Sight was in trouble. He says the lack of clarity about the company’s future “leaves you in a very, very vulnerable spot.” He says that all the Orion patients “were very much contemplating, ‘Are we going to get this removed now, before there’s no funding, before there’s no assurance?’ ” One of the six did choose to have the device removed in order to safely have an MRI scan. As for Spencer, he now uses the Orion sparingly, and plans to have the implant removed at the end of the study. “Had I known three years ago what I know now, I probably wouldn’t have signed up for it,” he says.
“If you provide excellent vision, there will be lots of patients. If you provide crappy vision, there will be very few.” —Daniel Palanker
Greenberg, the former Second Sight CEO, is now the
CEO and chairman of the Alfred Mann Foundation, which is involved in the Orion clinical trial. After the announcement of the proposed merger, he told Spectrum: “I still believe Orion has the potential to help many blind patients in a fiscally responsible way.”
The neurosurgeons involved in the work have been enthusiastic about the Orion technology. Daniel Yoshor, who implanted two Orion devices while chair of neurosurgery at Baylor College of Medicine, tells Spectrum that the technology was “an important first step.” Recently, he has experimented with stimulation patterns that give Orion patients more visual acuity, even enabling them to recognize large letters on a computer screen.
Recent experiments with new stimulation patterns for the Orion brain implant enabled users to see the shapes of letters.
Neurosurgeon Pouratian says that the 60-electrode brain implant is the most high-tech and precise neural implant to date, for any application. “From a technological standpoint, it’s pretty amazing in terms of its capabilities,” he says.
A much larger clinical trial would be required to gain FDA approval of the Orion. However, in the days after the merger announcement, Nano Precision Medical CEO Mendelsohn told
Spectrum that the merged company was “not committing to any sort of timeline with the Orion,” and emphasized that its priority will be NPM’s drug-delivery device. The new company will also “develop some strategic options for what makes sense going forward with the Orion technology,” Mendelsohn says.
Second Sight may have given up on its retinal implant, but other companies still see a need—and a market—for bionic vision without brain surgery. Paris-based Pixium Vision is conducting European and U.S. feasibility trials to see if its Prima system can help patients with age-related macular degeneration, a much more common condition than retinitis pigmentosa.
Daniel Palanker, a professor of ophthalmology at Stanford University who licensed his technology to Pixium, says the Prima implant is smaller, simpler, and cheaper than the Argus II. But he argues that it is Prima’s superior image resolution that will make Pixium Vision a success. “If you provide excellent vision, there will be lots of patients,” he tells Spectrum. “If you provide crappy vision, there will be very few.”
Some clinicians involved in the Argus II work are trying to salvage what they can from the technology.
Gislin Dagnelie, an associate professor of ophthalmology at Johns Hopkins University School of Medicine, has set up a network of clinicians who are still working with Argus II patients. The researchers are experimenting with a thermal camera to help users see faces, a stereo camera to filter out the background, and AI-powered object recognition. These upgrades are unlikely to result in commercial hardware today but could help future vision prostheses.
Failure is an inevitable part of innovation. The Argus II was an innovative technology, and progress made by Second Sight may pave the way for other companies that are developing bionic vision systems. But for people considering such an implant in the future, the cautionary tale of Argus patients left in the lurch may make a tough decision even tougher. Should they take a chance on a novel technology? If they do get an implant and find that it helps them navigate the world, should they allow themselves to depend upon it?
Abandoning the Argus II technology—and the people who use it—might have made short-term financial sense for Second Sight, but it’s a decision that could come back to bite the merged company if it does decide to commercialize a brain implant, believes Doerr.
“Who’s going to swallow their marketing for the Orion?” he says. Doerr is glad he has Second Sight’s technology in his retina instead of his brain tissue. “If it has to come out, it’s going to be bothersome,” he says, “[but] nobody is messing with my brain.”
NPM’s Mendelsohn says the merged company will explore its options with Orion and tells
Spectrum that “there’s a genuinely strong desire to try to find the best strategic option to bring [the Orion] technology to patients.” If development of the neural implant does go ahead, at least the new company will have the benefit of 20/20 hindsight. “We spend a lot of time thinking about the learnings from the Argus,” says Mendelsohn, “and how not to repeat going forward.”
From Your Site Articles
Related Articles Around the Web