11th January 2022
First heart transplant from a pig to a human
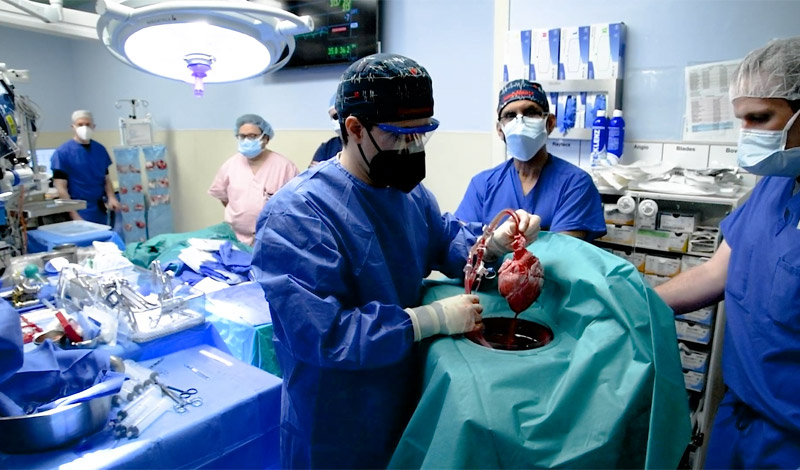
In a world medical first, a 57-year-old patient with terminal heart disease received a successful transplant of a genetically-modified pig heart and is still doing well four days later.
The University of Maryland Medical Center (UMMC) performed the historic surgery seen here, which demonstrated for the first time that a genetically-modified animal heart can function like a human heart without immediate rejection by the body. The patient, David Bennett, a Maryland resident, is being carefully monitored over the coming days and weeks to determine whether his transplant provides lifesaving benefits. Prior to this, he had been deemed ineligible for a conventional heart transplant at UMMC as well as at several other leading transplant centres that reviewed his medical records.
“It was either die, or do this transplant. I want to live. I know it’s a shot in the dark, but it’s my last choice,” said Mr. Bennett, a day before the surgery. He had been hospitalised and bedridden for the past few months. “I look forward to getting out of bed after I recover.”
The Food and Drug Administration (FDA) granted emergency authorisation for the surgery through its expanded access provision. This is used when an experimental medical product – in this case, the genetically-modified pig’s heart – is the only option available for a patient faced with a serious or life-threatening medical condition.
Scientists “knocked out” three genes – responsible for rapid, antibody-mediated rejection of pig organs by humans – in the donor pig. They then inserted six human genes responsible for immune acceptance of the pig heart into the genome. Lastly, they silenced one additional gene in the pig to prevent excessive growth of the pig heart tissue, making a total of 10 unique gene edits in the donor pig.
The potential for animal organs in so-called xenotransplantation has been discussed for many years. Surgeons have already transplanted pig heart valves, and in October 2021 a team in New York transplanted a pig’s kidney. Sadly, the recipient of that kidney had suffered brain death, with no hope of recovery. Mr Bennett’s operation is the biggest milestone in the field to date and could allow him to continue with his life.

David Bennett (third from left) and family.
“This was a breakthrough surgery and brings us one step closer to solving the organ shortage crisis. There are simply not enough donor human hearts available to meet the long list of potential recipients,” said Bartley Griffith, MD, who surgically transplanted the pig heart into the patient and is Distinguished Professor in Transplant Surgery and Director of the Cardiac Transplant Program at UMMC. “We are proceeding cautiously, but we are also optimistic that this first-in-the-world surgery will provide an important new option for patients in the future.”
“This is the culmination of years of highly complicated research to hone this technique in animals with survival times that have reached beyond nine months,” said Muhammad M. Mohiuddin, Professor of Surgery, who established the Cardiac Xenotransplantation Program with Dr. Griffith and serves as its Scientific Director. “The successful procedure provided valuable information to help the medical community improve this potentially life-saving method in future patients.”
About 107,000 Americans are currently waiting for an organ transplant, and more than 6,200 patients die each year before getting one, according to the federal government’s organdonor.gov. Xenotransplantation could potentially save thousands of lives but does carry a unique set of risks, including the possibility of triggering a dangerous immune response. These responses can trigger an immediate rejection of the organ with a potentially deadly outcome to the patient.
Revivicor, a regenerative medicine company based in Blacksburg, Virginia, provided the genetically-modified pig to the xenotransplantation laboratory at UMMC. On the morning of the transplant surgery, the surgical team, led by Dr. Griffith and Dr. Mohiuddin, removed the pig’s heart, and placed it in the XVIVO Heart Box – a perfusion device that keeps the heart preserved until surgery.
The physician-scientists also used a new drug, alongside conventional anti-rejection drugs, which are designed to suppress the body’s immune system and prevent it from rejecting the foreign organ. The new drug used is an experimental compound made by Kiniksa Pharmaceuticals.
“This is truly a historic, monumental step forward. While we have long been at the forefront of research driving progress toward the promise of xenotransplantation as a viable solution to the organ crisis, many believed this breakthrough would be well into the future,” said Bert O’Malley, MD, President and CEO, University of Maryland Medical Center. “I couldn’t be more proud to say the future is now. Our skilled team of physician-scientists will continue to advance and adapt medical discovery for patient care that could offer a lifeline for more patients in dire need.”
“As a cardiothoracic surgeon who does lung transplants, this is an amazing moment in the history of our field. Decades of research here at Maryland and elsewhere have gone into this achievement,” said Christine Lau, Surgeon-in-Chief at UMMC. “This has the potential to revolutionise the field of transplantation by eventually eliminating the organ shortage crisis.”
—
• Follow us on Facebook
• Follow us on Instagram
• Join us on Reddit
• Follow us on Twitter
• Subscribe to us on YouTube
Comments »